Aras PLM
Our advanced PLM solution suite helps global organizations achieve and maintain a wide range of environmental, industry and geographical standards, best practices and other initiatives.
Whether your focus is project management and process improvement, product development and manufacturing, or quality and safety, Aras offers the support you need to cost-effectively manage and document your products and processes, and provide the required reports and analyses to ensure compliance.
Aras standards and best practices capabilities include advanced configuration and change management, closed-loop workflows, project and program management, revision and version control, traceability and corrective actions, electronic signature, quality management and planning, and comprehensive business analytics.
Project Management
Aras Program Management (PM) addresses the issues of Project Integration, Scope Management, Time Management, Resource Management and Communication Management. Other Innovator Solutions, such as Quality Planning, address Quality and Risk Management, while Product Engineering addresses Procurement and Cost Management. The standard solutions can be extended to integrate Project Management with other business processes.

Product Engineering
Aras Poduct Engineering is a business ready solution for product definition and engineering change management across your company and with suppliers and customers.
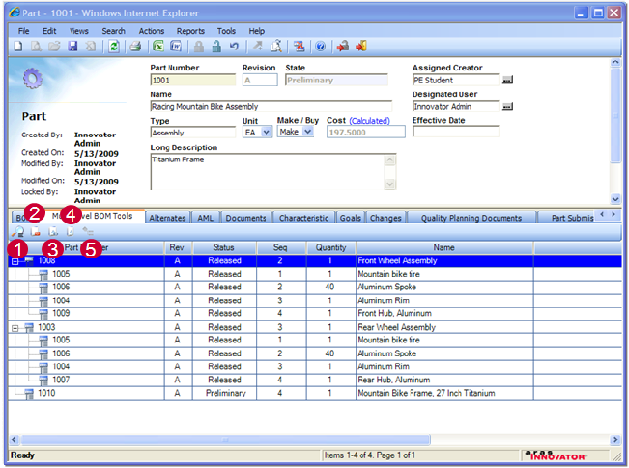
- Visibility into key development indicators increases engineering effectiveness and provides a basis for continuous improvement
- Manage product definition processes securely online to develop high quality products on time
- Engineering change management reduces cycle times and streamlines business operations
- Reuse of product and process information increases productivity and controls costs
Quality Planing
Aras Quality Planning provides enterprise organizations with comprehensive tools and processes to manage risk, improve quality and attain environmental, regulatory, safety, medical and other forms of compliance.
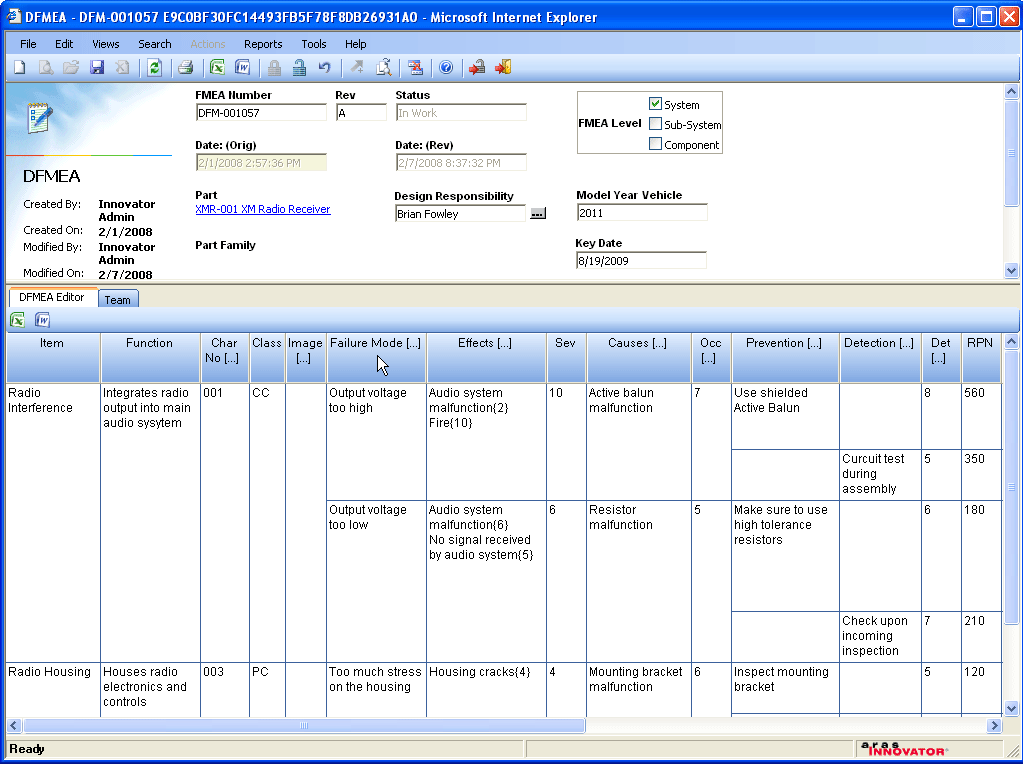
- Use Design for Six Sigma [ DFSS ] tools to control risks for greater profits and avoid potential catastrophic impact on costs and revenues by mitigating critical product risks before issues occur in the field.
- Closed-loop FMEA processes and action item accountability achieve compliance with regulatory requirements and industry-specific quality systems standards including TS/16949, AS9100, FDA QSR, and more.
- Define step-by-step process flows and integrate directly to FMEAs and Control Plans
- Secure online collaboration with customers, suppliers, and outsourced manufacturers
- Change control workflows enable compliance for ISO documents and specifications
- Manage Libraries of Critical Characteristics, Failure Modes, Effects, and Control Mechanisms
- Dashboards, Scorecards, KPI Metrics & Reports